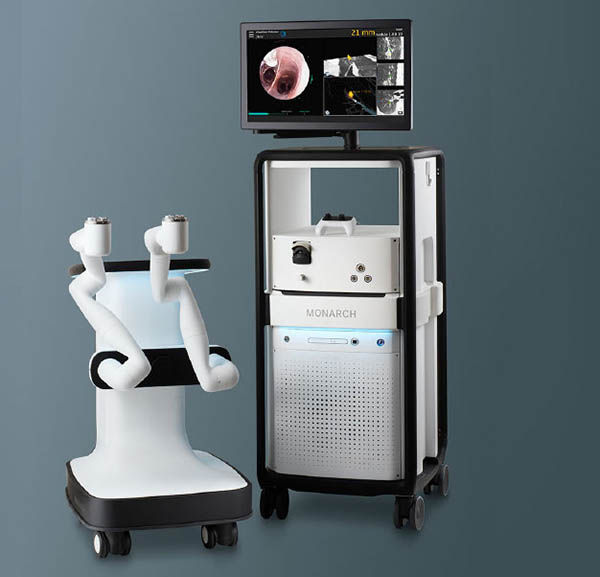
Auris Health Inc. this week announced that its surgical robotic system received 510(k) clearance from the U.S. Food and Drug Administration for bronchoscopy and urology procedures. Following this approval, it expects to start a first-in-human clinical study later this year for endourologic applications.
Redwood City, Calif.-based Auris Health is a subsidiary of Ethicon, which is a Johnson & Johnson medical technology company. It claimed that the FDA's clearance makes MONARCH the first and only flexible surgical robotics system that has gained approval for both urological and bronchoscopy surgeries.
Auris added that MONARCH was designed to enable urologists to reach and visualize areas within the kidney with precision and control.
MONARCH has been in tests since 2018
Since 2018, Auris Health said doctors have used MONARCH to perform robotically assisted bronchoscopy procedures and reach small, hard-to-reach peripheral lung nodules at an earlier stage and with greater precision than ever before.
“This latest FDA 510(k) clearance for MONARCH delivers on our vision to extend the robotic platform’s capabilities across multiple specialties, enabling hospital systems to target two disease states using one device,” said Vladimir Makatsaria, company group chairman of Ethicon.
“At Ethicon, we’re committed to driving meaningful innovation that elevates the standard of care for patients and delivers improved solutions that address important clinical challenges for our customers,” he added.
System designed to streamline urological procedures
The company said the MONARCH system provides urologists with one platform that supports both ureteroscopic and percutaneous nephrolithotomy procedures (PCNL). Ureteroscopic procedures are the most common surgical stone procedures globally but become increasingly challenging as stone size increases.
“With this FDA clearance, MONARCH is poised to aid physicians in their goals of reducing overall retreatment and complication rates,” said Dr. Jaime Landman, chairman of the department of Urology at University of California Irvine. “Furthermore, as physicians strive to elevate the standard of care and improve outcomes, MONARCH supports them in their primary goal of rendering patients stone free in a single procedure.”
Surgical robots offer less-invasive options
PCNL procedures have demonstrated superior stone clearance for patients with larger kidney stones, yet they represent only 7-8% of stone procedures conducted in the U.S. today, the company claimed.
Numerous barriers hinder more frequent use of PCNL, but the company said the MONARCH was designed to make surgeries minimally invasive.
“MONARCH reduces the complexity of gaining high-quality percutaneous access and aids stone clearance efficiency through simultaneous fragmentation and suctioning of stones with robotic assistance,” said Dr. Mihir Desai, professor of clinical urology at the University of Southern California.
“With this platform, many urologists may be willing to expand their practice to include percutaneous access and PCNL procedures, thereby increasing patient access to more effective treatments closer to home,” Desai said.
Article topics
Email Sign Up