Paul Cataford, Titan Medical Inc.'s interim president and CEO, said he is laser-focused on getting U.S. Food and Drug Administration clearance for Enos, the company’s single-access robotic surgical system.
The goal is to offer it for general gynecological procedures by early 2025, he said, with the qualifier that the company still has to go through a lot of regulatory hoops to get there.
“It’s a multistep process that takes a long time and a lot of focus,” he said. “That’s what the board and the senior leadership team are really focused on, making sure we stick to that timeline.”
An easier to use, more mobile system
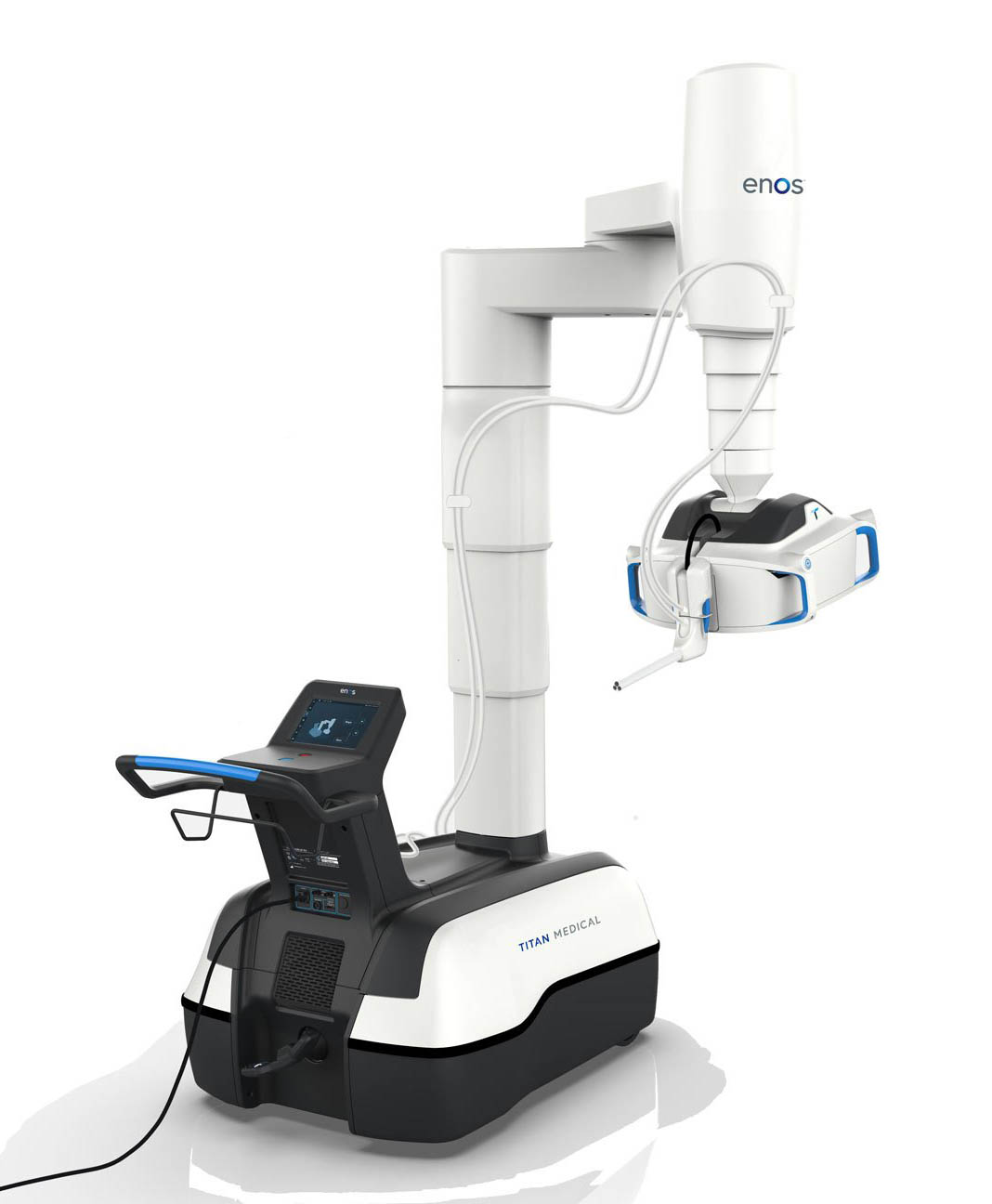
The surgical robot is made up of two main parts and is designed to have lower operating costs and a smaller footprint than traditional options.
Enos is also intended to be less invasive, since doctors will only need to make one incision to complete a procedure using the single-access system.

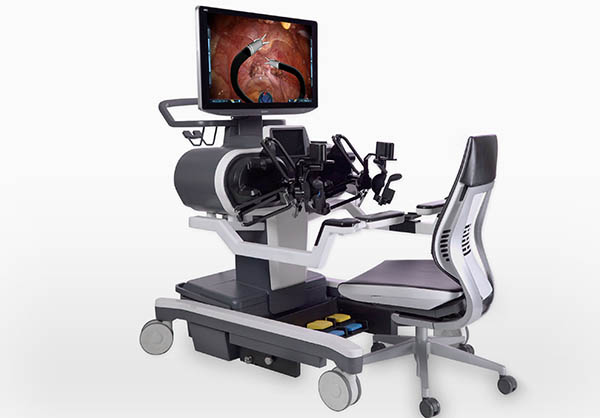
The surgeon’s workstation looks somewhat like a gaming PC setup and includes a high-definition 3D display, hand and foot controllers, swivel wheels, and a chair.
The mobile patient cart includes two small mobile articulating arms, two lighted cameras, a 2D high-definition camera, and an articulating 3D high-definition camera.
“Our goals are to enable better patient outcomes by allowing faster recovery times and less trauma and scarring, and improved clinical performance, operating room efficiency, and favorable hospital economics,” said the company’s website.
Titan Medical to start human trials next year
The system is particularly well suited for gynecological surgeries, according to Chris Seibert, vice president of upstream marketing at Titan Medical.
That’s why the Toronto-based company is seeking clearance for those types of procedures first. Cataford said the company will be completing human clinical trials for gynecological procedures starting next year at four sites involving 40 to 60 patients.

Seibert said the system works well for such procedures because the surgeons have more space within that region of the body “so you have the ability to move things around to get the optimum view.”
45 different surgeries successfully completed
But the Enos system is capable of doing more than just gynecological procedures, Seibert said. Titan Medical said it has completed over 70 labs, and the system has helped physicians perform more than 45 types of procedures. Much of the feasibility work was done in 2017 and 2018, according to Seibert.

Cataford said once the company has been given De Novo regulatory approval for gynecological indications, it can submit 510k filings to the FDA, which will help it gain approval for other indications, he said.
Titan Medical said it hopes to offer its technology for urological and kidney procedures. From there, the next big target would be general surgery such as gallbladder removal and hernia repair.
“There are a number of [areas] we can take it, and we’ve tried all of these, and it’s performed even better than we anticipated,” Seibert said.
'Shrink everything down'
One of the Enos System’s benefits is that it allows surgeons to have more control in small spaces within the body, said Seibert.
Doctors also appreciate that they can complete surgeries through a single incision, Seibert said.
“What we are able to do is shrink everything down,” he said. “The incision used to be 10 to 12 in. Now it’s down to 2 in., and you are able to get full functionality of the human hand through that tiny incision.”
Surgeons want cheaper systems that are smaller and more comfortable to use, as well as easier to control and navigate, he added.
Titan Medical in a small group
Titan Medical is among several companies in the medical space offering single incision robotic-assisted surgeries. Many robotics surgeries involve multiport systems.
“Typically, a multiport case is going to have a minimum of four incisions, we shrink that to one to two to the max,” Seibert said.
A few of the company’s single-port robotic surgical system competitors include Vicarious Surgical and Intuitive Surgical.
Seibert said the company welcomes the competition since it will drive down costs and propel innovation.
“With us and others coming into the field, the surgeon and the patient are going to be the winners,” he said.
About the Author
Follow Robotics 24/7 on Linkedin
Article topics
Email Sign Up