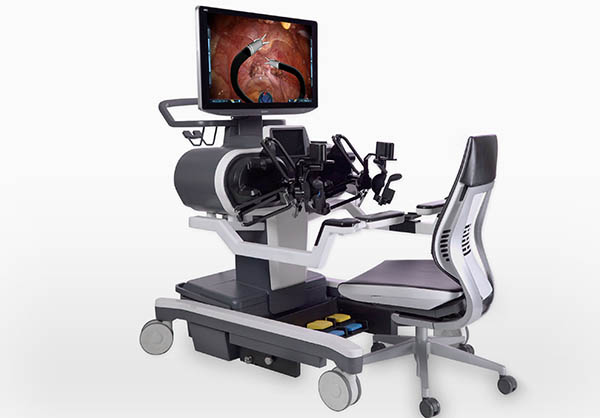
Titan Medical Inc. last week announced the publication of an international patent application that discloses details concerning “dexterous endoscope technologies for use with single access robotic-assisted surgery [RAS] systems.”
The Toronto-based company said the technologies will provide a greater field of view than what is offered with current endoscope tools. The company is currently going through the U.S. Food and Drug Administration process for its Enos surgical robotic system.
“Technology described in the patent application could be utilized in a variety of single access RAS systems, including those designed to support three instruments that are simultaneously positionable within an insertion conduit,” the company said.
Titan Medical said it is an innovative leader in the surgical robotics space
In an interview with Robotics 24/7 earlier this year, Vance said while the company is navigating the FDA process to get its Enos medical systems cleared for use, it is also working to continuously innovate. That’s reflective of his statement following the publication of this patent application.
“It was important for us to share the publication of this patent application as it highlights our continued commitment to be an innovation leader in single access RAS, which includes but also extends beyond the current development of the Enos surgical system,” said Cary Vance, president and CEO of Titan in a statement.
“While we are focused on the development, clinical, and regulatory activities for the Enos system, we are committed to continuing to advance the surgical experience for patients, surgeons, and hospitals through the research and development of RAS technology components and systems,” he added. “Filing patent applications on our inventions remains an important step in protecting the value we continue to generate, including those designed for next-generation technologies and systems.”
The company said it has a portfolio of over 220 pending and issued patents for “various aspects of single-access RAS including the Enos system’s surgeon workstation, patient cart, dexterous articulating instruments, ergonomic hand-controllers, enhanced vision systems, advanced control software, and instinctive surgeon overlays for providing interoperative feedback.”
“This coverage of single-access RAS, and in particular the Enos system, along with coverage of more general aspects of RAS technologies, may provide the company with multiple intellectual property options including further protecting its RAS technologies, and the potential to further license its technologies and assistance in securing a competitive commercial pathway,” it added.
The patent application is numbered: PCT/CA2022/050392, which the company anticipates pursuing in the United States and other key markets under the Patent Cooperation Treaty (PCT).
Article topics
Email Sign Up