Every year, 5 million Americans undergo abdominal soft-tissue procedures, 90% of which do not include access to robot-assisted surgery, or RAS. Virtual Incision Corp. this week announced the completion of its clinical study for an Investigational Device Exemption, or IDE, from the U.S. Food and Drug Administration.
The study was designed to evaluate Virtual Incision's MIRA, a miniaturized, robot-assisted surgical system for use in bowel-resection procedures. The company said it was an important step toward bringing new technologies to hospitals and surgical robotics programs, regardless of the site of care.
“Completing MIRA’s IDE clinical study is a critical milestone in our journey to making RAS more accessible,” stated John Murphy, president and CEO of Virtual Incision. “Currently, less than 10% of the 90,000 operating rooms in the U.S. are equipped with mainframe RAS systems.”
MIRA designed for ease of use
Virtual Incision claimed that MIRA is “the world’s first miniaturized RAS system.” The company said the device is designed to offer the benefits of robotic surgery during bowel-resection procedures without the bulk or logistical inefficiencies of traditional, mainframe robots.
MIRA weighs about 2 lb. and offers internal triangulation with shoulders, arms, and infinite wrist roll inside of the body. It can be used in any operating room, so a dedicated mainframe room is unnecessary, claimed Virtual Incision.
“With its drape- and dock-free design and portability, MIRA is quick to set up, clean up, and move between cases,” said the company. “Its conveniently accessible design positions it to be used as a standalone system or a complementary tool for facilities that already own a mainframe. With MIRA, every operating room is RAS-ready.”
The surgical cases were completed at three hospitals across the country. The study continued to follow patients after their procedures. Virtual Incision plans to correlate and submit the complete data to the FDA as part of its De Novo request for market authorization.
The company claimed that it is the first surgical robotics developer to complete a U.S. IDE study to support a De Novo request in bowel resection.
“The investigators are very encouraged by our experiences trialing the MIRA Surgical System,” said Michael A. Jobst, an MD and colorectal surgeon. “Across the sites, we’ve seen MIRA efficiently integrate into existing RAS programs and witnessed how it is mobile enough for use in any operating room.”
“Some sites have even completed multiple cases in a single day,” said Dr. Jobst. “We are eager to fulfill the clinical requirements of the study in hopes that MIRA can help expand RAS access to more patients in the future.”
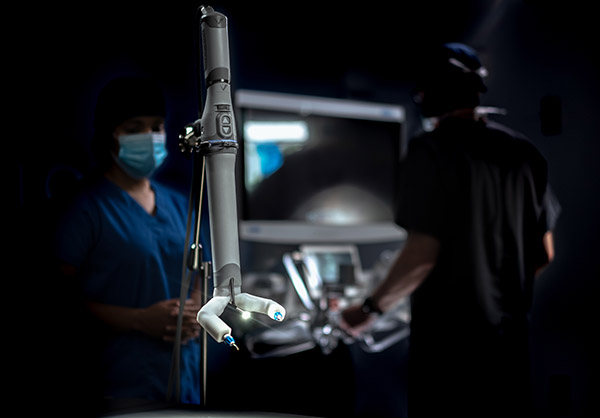
Virtual Incision aims to simplify robotic surgery
Lincoln, Neb.-based Virtual Incision said that by simplifying robot-assisted surgery, more patients and their surgeons can benefit from it. The company holds more than 200 patents and patent applications and said it is working “to make every operating room RAS-ready.”
“Beyond the incredible progress of the industry pioneer, it’s still in the early days of the adoption of soft-tissue surgical robotics,” said Murphy. “Our ultimate goal is to develop world-class miniature RAS devices with the required strength and dexterity to enable positive clinical outcomes for a broad range of procedure types.”
The global market for surgical robots will grow thanks to improving technical capabilities and falling prices, according to Research and Markets. It predicted growth in gynecological and neurological procedures, software and services, and the Asia-Pacific market.
If authorized by the FDA, MIRA’s miniaturized, strong, and easy-to-use design would increase the overall availability of RAS, said Virtual Incision. It has the potential to integrate into any facility or operating room and complement existing surgical robots, the company added.
In addition, MIRA could expand into new sites of care and geographies as a standalone. Virtual Incision said it aims to increase patient access through a clinically, operationally, and economically sound platform available to all providers.
Article topics
Email Sign Up















